HOW DOES NITROGEN HELP PLANTS GROW?
Importance of Nitrogen in Soil & Plants
Nitrogen
Nitrogen is one of the macronutrients which is required in large amount for plant metabolism and growth act as a primary nutrient for plants. It is absorbed in Ammonium (NH4+), Nitrate (NO3-) ions forms. Nitrogen is the element which í not directly available to plants from atmosphere and earth's crust.
Importance of Nitrogen in Soil
- In soil atmospheric nitrogen is a major source of nitrogen. In the atmoshere, it exists in N form and must be converted before it becomes useful in the soil.
- Rhizobia is a bacterium that ìmects the roots ò and receives much food enerrgy from, legume plants can fix much more nitrogen per year. The quantity ò nitrogen fixed by Rhizobium bacteria exceeds that needed by the microbes them selves for use by the host legume plant.
- Nitrogen has three forms as soil organic nitrogen compounds, ammonium (NH4+) ions and nitrate (NO3-) ions. In soil, nitrogen is available to plants it needs microorganism to convert so that they can available for plants. The nitrogen in soil minerals í generally slow and contributes only slightly to nitrogen in the soil. The available nitrogen is in the inorganic forms NH4+ and NO3- these forms are mostly available in the soil.
Ammonium ions bind to the soil in negatively charged cation exchange complex (CEC). Nitrate ions do not bind to the soil solids because the have nagative charges, but exist disolved in the soil water, or precipitated as soluble salts under dry conditions.
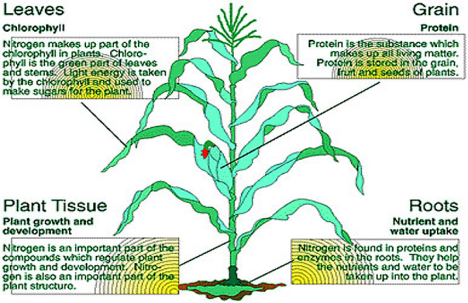
Importance of Nitrogen in Plants
- Nitrogen is a major component ò chlorophyll, the compound by which plants use sunlight energy for photosynthesis to produce sugars form water and carbon dioxide.
- It plays an importtant in soil fertility management so it has higher concentration compared to carbon, hydrogen and oxygen.
- Nitrogen omparts vigorous vegetative growth dark green colour to plants.
- Nitrogen is an enery-transfer compound, sush as ATP (adenosine triphosphate). ATP allows cells to conserve and use the energy released in metabolism in plants.
- Nitrogen is major component of amino acids.
- It is an esential constituent of protein and present in many other compounds of physiological importance in plant metabolism.
- Nitrogen produces early growth and also results in delay in maturity in plants.
- A complex group of protein such as Nucleoprotein involved in the development and hereditary process. It is a significant component of nucleic acids sush as DNA (Deoxyribonucleic acid), RNA (Ribonucleic acid) the genetic material thet allows cells to grow and reproduce.
- The chief source of Nitrogen in the soil because plant absorb it either in the form of nitrate or Ammoniacal salt and it governs the utilization of potassium, phosphorus and other elements.
- When the temperature is lower than higher is the N content due to more organic matter addition and slow rate of decomposition.
- Improves th quality of leafy vagetables and fodders and also in creasing protein content some becteria and hêtrocyst containing blue-green algae fix nitrogen of the atmosphere which can be utilised by plants.
- Ammonium (NH4+) fixcation increases with in crease in 15 the content particularly 2:1 type of clay minerals linke vermiculite, fine-grained mica and smectite.
- Fixation of ammonium ion (NH4+) is geneally higher in subsoil due to higher clay content and a lower rate of nitrification.
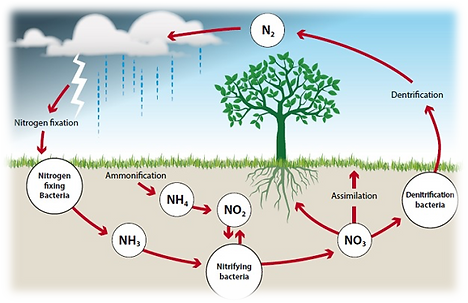
NITROGEN CYCLE
- The nitrogen cycle is a biological process through which nitrogen is converted into ammonium ion and nitrates ions by the atmosphere to soil to the organism and again into the ecosystem.
- It exists in two forms organic forms and inorganic forms. Organic forms involve animal waste, manures, residue etc the all go through the decomposition process. Inorganic forms involve symbiotic bacteria which converts nitrogen into the usable form so that plants can absorb.
- It has several processes sushas Nitrogen fixation, Nitrification, Assimilation, Ammonification and Denitrification.
Nitrogen Fixation
- The first step in the Nitrogen cycle.
- It converts Atmospheric nitrogen (N2) which is insert form in to usable ammonia (NH3).
- Atmospheric nitrogen source is from the atmosphere, surface waters through precipitation, rainfall.
- It undergoes changes into two nitrogen atoms and combines with hydrogen to form ammonia.
- Symbiotic bacteria involved in this process is Diazotrophs and azotobacter, rhizobium also has a major role in this process.
Types of Nitrogen fixation involved are
- Atmosphere fixation: Natural phenomenon (energy ò lightning).
- Industrial nitrogen fixation: Man-made alternative (Urea).
- Biologicla nitrogen fixation: Bacteria like Rhizobium and blue-green algae fixes nitrogen components in soil.
NITRIFICATION
- The ammonia is converted into nitrate by oxidation of ammonia with help of Nitrosomonas bacteria and later produce nitrites into nitrates by Nitrobacter
- Nitrification reaction:
2NH4+ + 3O2 → 2NO2– + 4H+ + 2H2O
2NO2– + O2 → 2NO3–
Assimilation
The process of converting nitrogen compounds such as ammonium ion, nitrate ion, nitrite ion is used for the formation of plants and animal protein.
Ammonification
- When organic material decomposes the nitrogen present in these matters are released into the soil and converts that material into ammonium.
- These are decomposed by bacteria or fungi in soil and produce ammonia which is used for biological processes.
Denitrification
Denitrification is the final step in the Nitrogen cycle.
The process in which nitrate (NO3-) converts into Nitrogen (N) is called Denitrification and in this process, nitrate to gain oxygen and gives out free nitrogen as a by-product.
Bacteria involved in this process are Clostridium and pseudomonas bacteria.
NƠI MUA CÁC SẢN PHẨM PHỤC VỤ NÔNG NGHIỆP:
CÔNG TY CỔ PHẦN XUẤT NHẬP KHẨU PHÂN BÓN QUỐC TẾ ASIA
VPĐD: Tầng 14, Tòa Nhà HM Town, 412 Nguyễn Thị Minh Khai, Phường 05, Quận 3, TP Hồ Chí Minh
Điện thoại: (02963) 63 66 99 hoặc 0838 22 99 77.